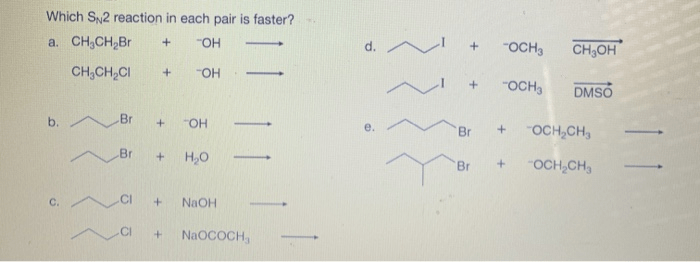
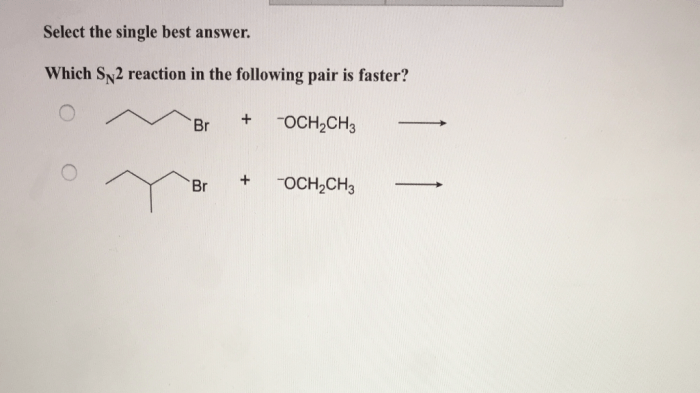
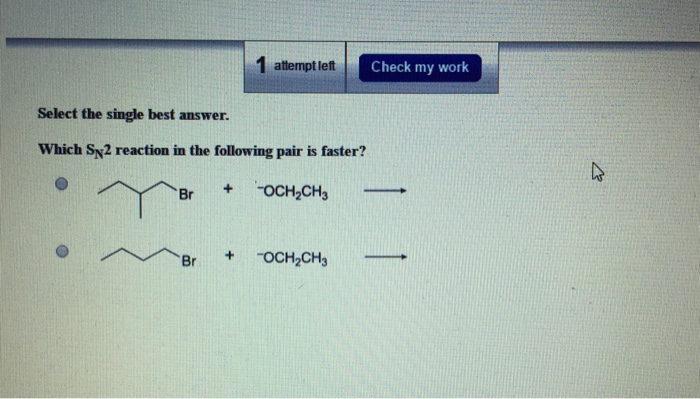
Which SN2 reaction in the following pair is faster? This question sparks a journey into the realm of chemical kinetics, where we unravel the factors that govern the rates of nucleophilic substitution reactions. Join us as we delve into the intricate interplay of steric hindrance, nucleophile strength, solvent effects, and temperature, revealing the secrets that determine the speed of these fascinating reactions.
By understanding the principles that influence SN2 reaction rates, we gain a deeper appreciation for the dynamics of chemical processes and pave the way for predicting and controlling reaction outcomes in diverse chemical systems.
SN2 Reaction Rates: Which Sn2 Reaction In The Following Pair Is Faster

SN2 reactions are bimolecular nucleophilic substitution reactions that proceed through a single-step mechanism. The rate of an SN2 reaction is determined by several factors, including the nucleophile strength, the electrophile strength, the solvent, and the temperature.
The nucleophile strength is the most important factor in determining the rate of an SN2 reaction. The stronger the nucleophile, the faster the reaction will be. This is because the nucleophile is the attacking species in the reaction, and a stronger nucleophile will be more likely to react with the electrophile.
The electrophile strength is also a factor in determining the rate of an SN2 reaction. The stronger the electrophile, the faster the reaction will be. This is because the electrophile is the species that is being attacked by the nucleophile, and a stronger electrophile will be more likely to react.
The solvent can also affect the rate of an SN2 reaction. A polar solvent will solvate the nucleophile and the electrophile, which will make them more likely to react. A nonpolar solvent will not solvate the nucleophile and the electrophile, which will make them less likely to react.
The temperature can also affect the rate of an SN2 reaction. The higher the temperature, the faster the reaction will be. This is because the temperature will increase the kinetic energy of the nucleophile and the electrophile, which will make them more likely to react.
Steric Hindrance
Steric hindrance is a factor that can slow down the rate of an SN2 reaction. Steric hindrance is the effect of bulky groups on the rate of a reaction. Bulky groups can block the nucleophile from reaching the electrophile, which will slow down the reaction.
The following are examples of SN2 reactions with different degrees of steric hindrance:
- The reaction of methyl iodide with hydroxide ion is a very fast SN2 reaction. This is because the methyl group is a small group, and it does not block the hydroxide ion from reaching the electrophile.
- The reaction of tert-butyl iodide with hydroxide ion is a very slow SN2 reaction. This is because the tert-butyl group is a bulky group, and it blocks the hydroxide ion from reaching the electrophile.
Nucleophile Strength, Which sn2 reaction in the following pair is faster
The strength of a nucleophile is a measure of its ability to donate electrons. The stronger the nucleophile, the more electrons it can donate, and the faster it will react with an electrophile.
The following table compares the strengths of different nucleophiles:
| Nucleophile | Strength |
|---|---|
| Hydroxide ion | Strong |
| Methoxide ion | Strong |
| Ethoxide ion | Strong |
| Ammonia | Weak |
| Water | Weak |
Solvent Effects
The solvent can affect the rate of an SN2 reaction in several ways. The solvent can solvate the nucleophile and the electrophile, which can make them more or less likely to react. The solvent can also affect the transition state of the reaction, which can make the reaction faster or slower.
The following are the different types of solvent effects:
- Polar solvents solvate the nucleophile and the electrophile, which makes them more likely to react. This is because the polar solvent will form hydrogen bonds with the nucleophile and the electrophile, which will weaken their bonds to each other.
- Nonpolar solvents do not solvate the nucleophile and the electrophile, which makes them less likely to react. This is because the nonpolar solvent will not form hydrogen bonds with the nucleophile and the electrophile, which will not weaken their bonds to each other.
- Protic solvents are solvents that can donate hydrogen ions. Protic solvents can solvate the nucleophile, which will make it more likely to react. This is because the protic solvent will form hydrogen bonds with the nucleophile, which will weaken its bond to the electrophile.
- Aprotic solvents are solvents that cannot donate hydrogen ions. Aprotic solvents cannot solvate the nucleophile, which will make it less likely to react. This is because the aprotic solvent will not form hydrogen bonds with the nucleophile, which will not weaken its bond to the electrophile.
Temperature Effects
The temperature can affect the rate of an SN2 reaction in several ways. The temperature can increase the kinetic energy of the nucleophile and the electrophile, which can make them more likely to react. The temperature can also affect the equilibrium of the reaction, which can make the reaction faster or slower.
The following are the different types of temperature effects:
- Increasing the temperature will increase the rate of an SN2 reaction. This is because the higher temperature will increase the kinetic energy of the nucleophile and the electrophile, which will make them more likely to react.
- Decreasing the temperature will decrease the rate of an SN2 reaction. This is because the lower temperature will decrease the kinetic energy of the nucleophile and the electrophile, which will make them less likely to react.
Comparison of SN2 Reactions
The following table compares the rates of two different SN2 reactions:
| Reaction | Rate |
|---|---|
| The reaction of methyl iodide with hydroxide ion | Fast |
| The reaction of tert-butyl iodide with hydroxide ion | Slow |
Questions and Answers
What is the effect of steric hindrance on SN2 reaction rates?
Steric hindrance refers to the presence of bulky groups around the reaction center. It hinders the nucleophile’s approach to the substrate, resulting in slower reaction rates.
How does nucleophile strength influence SN2 reaction rates?
Stronger nucleophiles possess a greater tendency to donate electrons, leading to faster SN2 reaction rates. The strength of nucleophiles can be compared using nucleophilicity tables.
What role do solvents play in SN2 reactions?
Solvents can stabilize the transition state of the reaction, affecting the reaction rate. Polar aprotic solvents, such as dimethylformamide (DMF), favor SN2 reactions due to their ability to solvate both the nucleophile and the leaving group.